Published: May 15, 2023
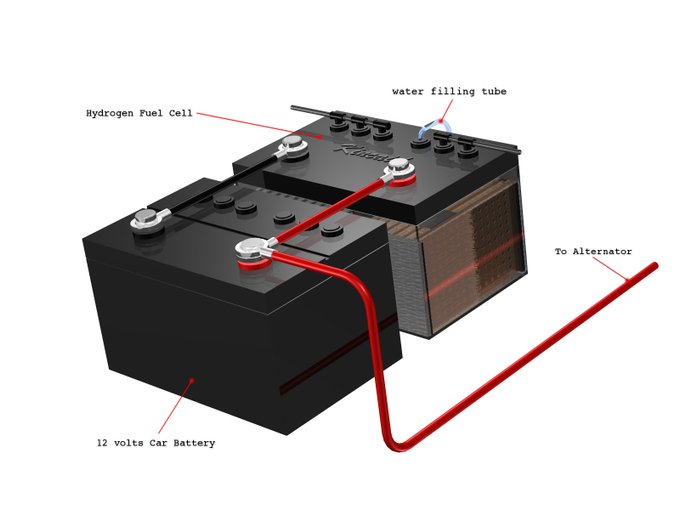
Clean hydrogen fuel can easily be produced to power gas combustion engines. Hydrogen (a natural gas) can easily be produced from water – tap water, sea water, drain water, any water. The fuel cell uses the mass produced 12 volt car battery and the alternator that are in every gas combustion engine vehicle to convert water into hydrogen gas fuel.
Water is H2O (hydrogen and oxygen) and water can easily be split (fissioned) into zero emission hydrogen gas using a simple method called electrolysis (cold fission).
In electrolysis of water a very small electrical current is passed between two electrodes submerged in water. Electrolysis of water will begin with a minimum of 1.2 volts and will increase in rate as the voltage is increased. Hydrogen gas accumulates at the cathode (negative electrode) and oxygen gas at the anode (positive electrode).
Sources of electrical current that can be used in the electrolysis of water – car battery (12 volts) or a solar panel.
In the article “Simple electronic device instantly increases solar panel output and efficiency by more than 700%” I, Paul W Kincaid, made and posted a video to demonstrate how to increase solar panel output and efficiency.
The solar panel’s 19 volts DC output was instantly increased to 166 volts DC. More than enough energy to power a H2O fuel cell and produce more than enough hydrogen fuel to power a gas combustion engine like a portable electrical power generator or fuel gas stoves, gas fireplaces, gas barbecues and gas home heating furnaces.
Hydrogen gas from water produces no toxic carbon emissions. It is a carbon free, environmentally friendly and unmetered fuel.
In 2008 I produced the following video and I posted it on YouTube Feb. 23, 2011 and on my PRESS Core website to demonstrate just how easy it is to produce clean hydrogen gas fuel from water using very little energy.
My H2O fuel cell was designed to use sea water. It can also use brine water from the flooded potash mine shafts in Sussex and Norton NB.
Very little energy was used to cold fission (the action of dividing or splitting something into two or more parts) water and produce hydrogen and oxygen gases. Just 4.5 volts DC was used. Most batteries of cell phones, digital cameras and tablets are recharged using a 5 volts USB charger. Therefore, a car’s 12 volt battery would produce more hydrogen gas fuel and a solar panel with an output of 166 volts (using method demonstrated in PRESS Core article “Simple electronic device instantly increases solar panel output and efficiency by more than 700%”) would produce much more hydrogen gas fuel and much more quickly.

Water covers 71% the Earth’s surface and the oceans contain 97.2% of Earth’s water. There are 343 quintillion gallons or 11 quintillion barrels of water in our oceans. Enough clean energy fuel to fuel the entire World for millenniums.
Not a drop of fresh drinking water or toxic chemicals are needed to recover this high grade, clean burning fuel. This hydrogen fuel produces no harmful by-products upon combustion. This hydrogen gas fuel has no environmental impact. Fresh drinking water isn’t contaminated. The soil isn’t contaminated, nor is the air, or oceans. When this hydrogen fuel is burnt it releases energy and the only bi-product left is desalinated water and pure clean oxygen.
Producing hydrogen gas fuel from sea water is achievable today. Sea water electrolysis (cold fission) reactor domes can be built on a nation’s ocean shores quickly and easily using available technology.
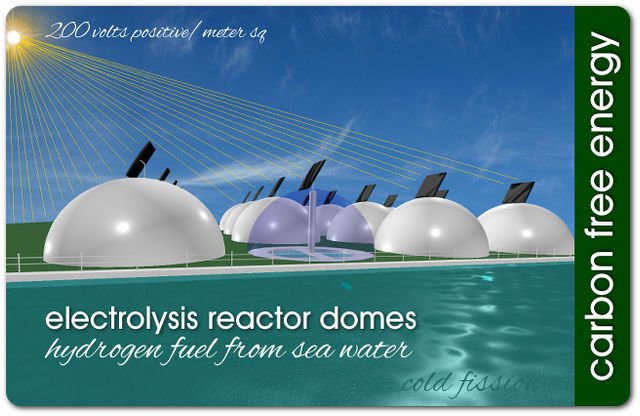
The electrolysis reactor domes can be made of recycled plastic. The free energy source needed to convert sea water into hydrogen gas is the Sun. Solar panels can harness this free energy and split sea water into hydrogen gas and oxygen. Submerged inlet pipes will keep the dome enclosed hydrogen fuel cells toped up with sea water 24/7.
